
It seems that there is a parallel between energy and.

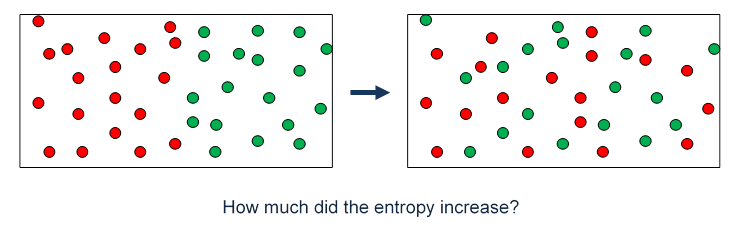
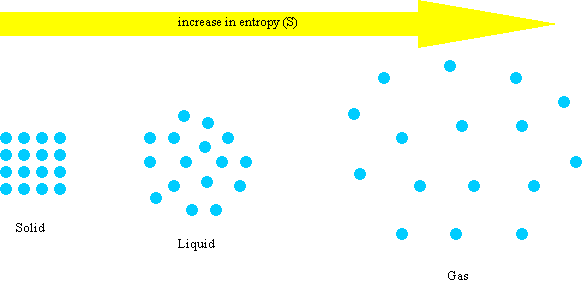
All spontaneous change occurs with an increase in entropy of the universe. These increases in entropy production will be due to the increase in heat production within the body. The universe tends toward increased entropy. A positive (+) entropy change means an increase in disorder. These results help us understand the mechanisms underlying the psychedelic state and, more generally, the pharmacological modulation of whole-brain activity. Entropy, S, is a state function and is a measure of disorder or randomness. You must consider each system on its own merits and there may be both increases and decreases happening together. Interestingly, at the whole-brain level, this reconfiguration was not well explained by 5HT2A-R density, but related closely to the topological properties of the brain’s anatomical connectivity. Equation (7.1) states that there is an entropy increase due to the increased volume that each gas is able to access. We also found that entropy changes were not uniform across the brain: entropy increased in some regions and decreased in others, suggesting a topographical reconfiguration mediated by 5HT2A-R activation. Our results reproduce the overall entropy increase observed in previous experiments in vivo, providing the first model-based explanation for this phenomenon. We sought to do this here by building upon a recent whole-brain model of serotonergic neuromodulation, to study the entropic effects of 5HT2A-R activation. Entropy can only be calculated it can never be observed directly. The increase in an entropy leads to an irreversible change in a system because some of the energy is expended as waste heat that limits the amount of work a system can perform. Thus, entropy measurement is a way of distinguishing the past from the future. Entropy never decreases for an isolated system.
This increase in the air must be more than the decrease in the water, because the whole systems entropy must increase. As one goes 'forward' in time, the second law of thermodynamics says, the entropy of an isolated system can increase, but not decrease. This happens because heat energy is transferred from the water to the surrounding air, therefore increasing the entropy of the air. However, no clear mechanistic explanation for this entropy increase has been put forward so far. Entropy is one of the few quantities in the physical sciences that require a particular direction for time, sometimes called an arrow of time. One of the most notable neurophysiological signatures of psychedelics, increased entropy in spontaneous neural activity, is thought to be of relevance to the psychedelic experience, mediating both acute alterations in consciousness and long-term effects. Abstract: Psychedelic drugs, including lysergic acid diethylamide and other agonists of the serotonin 2A receptor (5HT2A-R), induce drastic changes in subjective experience, and provide a unique opportunity to study the neurobiological basis of consciousness.


 0 kommentar(er)
0 kommentar(er)
